Receive the latest updates from Matrix Medical in real time to understand product information.
The following article is from Arterial Network, written by Ji Jiaying
Recently, Hangzhou Juzheng Medical Technology Co., Ltd. (hereinafter referred to as Juzheng Medical) successfully completed the pre market clinical trial of its independently developed "Coronary Artery Remodeling Catheter". This milestone not only marks a crucial step forward in the development of this "world first" product, but also signifies the phased validation of Juzheng Medical's innovation and transformation capabilities in the field of vascular intervention devices.
This prospective, multicenter, randomized controlled study, led by the Second Affiliated Hospital of Zhejiang University School of Medicine, aims to evaluate the safety and effectiveness of coronary artery remodeling catheters in the treatment of patients with coronary atherosclerotic heart disease.
As a novel clinical treatment pathway for vascular injury repair, the coronary artery remodeling catheter provides an innovative solution that combines "photochemical crosslinking technology+drug balloon technology": this product uses photochemical crosslinking technology to elastically reshape blood vessels, providing sustained natural support and achieving in-situ self-healing repair of the vessel wall, thereby reducing intervention on surrounding tissues. Its clinical application value mainly lies in the following three aspects:
Reduce damage during vascular intervention surgery
Inhibit the elastic contraction of the vascular wall and the formation of dissection
Maintain the natural anatomical structure and physiological characteristics of the vascular lumen
Academician Wang Jian'an commented on this: "The birth of the Ju Zheng Medical Coronary Reshaping Catheter marks a global breakthrough in the field of photochemical technology in China. At the same time, similar technologies in the United States have also obtained FDA's' Breakthrough Medical Device Certification ', but their research and development progress has fallen behind China. At present, the clinical enrollment of coronary artery remodeling catheters has been successfully completed, and we hope that this innovative achievement from China can benefit cardiovascular disease patients worldwide
01
Case sharing: Effective implementation of vascular recanalization without interlayer formation
It is reported that the 53 year old male patient who underwent this operation had a history of type 2 diabetes for more than 10 years. More than 10 months before surgery, a stent was implanted in the right coronary artery. Over 2 months ago, the patient experienced symptoms of dizziness and palpitations, without any discomfort such as chest pain, tightness, tightness, or blackness. For further diagnosis and treatment, the patient went to the hospital for treatment. The diagnosis results showed that the patient had primary coronary artery stenosis and had a history of hypertension, hyperuricemia, and renal dysfunction for several years.
■ Preoperative evaluation
Preoperative angiography showed diffuse long lesions in the middle segment of the anterior descending artery, with stenosis up to 80% and TIMI grade 3 blood flow; The stenosis in the distal segment of the circumflex branch is the heaviest at 80%, with TIMI grade 3 blood flow; The original stent in the middle section of the right coronary artery has unobstructed blood flow, mild intimal hyperplasia inside the stent, and TIMI grade 3 blood flow. After evaluation, the treatment team has decided to first treat the anterior descending artery disease and ensure good treatment before using the Zhengzheng Medical Coronary Reshaping Catheter to treat the patient's distal circumflex branch.
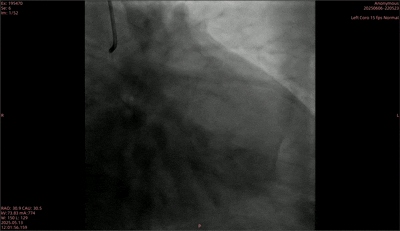
Preoperative
■ Surgical Treatment
During the surgery, two drug-eluting stents were first implanted in the anterior descending artery, and repeated imaging showed the narrowing was relieved. Subsequently, a pre expanded balloon with a size of 2.0mm × 15mm was used to pre expand the distal segment of the circumflex branch. After expansion, the diameter stenosis was 25%, and the blood flow was TIMI grade 3. However, a B-type dissection appeared at the lesion site after pre expansion. Subsequently, a 2.0mm × 30mm rectangular medical coronary artery remodeling catheter was used to dilate the blood vessels with a pressure of 6atm, and laser treatment was initiated simultaneously. In the end, the postoperative imaging results showed good dilation effect at the stenosis site, residual stenosis of 20%, disappearance of the original dissection, TIMI grade 3 blood flow, and successful completion of the surgery.

Postoperative
At present, some cases have completed follow-up, and the follow-up results are in line with expectations. It is expected that the coronary artery remodeling catheter will be submitted for registration approval and launched in 2026, bringing a new treatment option of "interventional non implantation" for cardiovascular disease patients.
As the main researcher of this clinical study, Professor Jiang Jun pointed out that "for complex and high-risk coronary heart disease patients, existing treatment methods often struggle to balance short-term efficacy and long-term safety, facing common problems such as vascular dissection and restenosis. Coronary artery remodeling catheters, with their unique dual mechanism of 'balloon dilation+in situ healing', can effectively suppress the risk of dissection and vascular rebound, providing a new treatment option for clinical practice
Jiang Jun, Chief Physician of the Department of Cardiology at the Second Affiliated Hospital of Zhejiang University School of Medicine
02
In situ support, efficient and seamless,
Assist in further upgrading the level of PCI treatment
With the acceleration of aging and the change of lifestyle, the incidence rate of coronary heart disease in China is rising. As the core means of coronary heart disease treatment, percutaneous coronary intervention (PCI) is showing a trend of rapid growth in the number of operations. According to the registration data of coronary intervention therapy (PCI) in Chinese Mainland released by the Quality Control Center of Coronary Intervention Technology of the National Health Commission, the total number of PCI operations in 2023 will be 1.63 million, an increase of 26.44% over 2022, a record high. In addition, the growth rate of 26.44% in PCI cases has also reached its highest level in history.
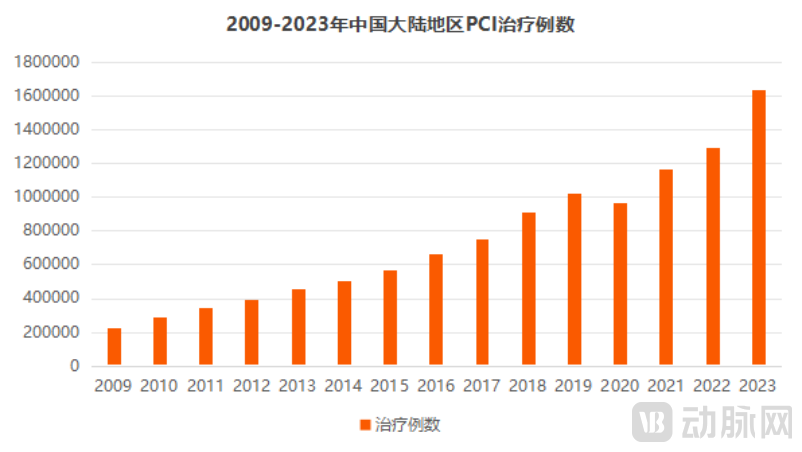
Data source: Physician's report, arterial network mapping
After more than 40 years of development, PCI technology has been continuously iterated and upgraded, gradually evolving from percutaneous coronary angioplasty (PTCA) and bare metal stent (BMS) implantation to the application of the new generation of drug-eluting stents (DES) implantation and drug-eluting balloons (DCB).
Among them, drug-eluting stents prevent lumen narrowing caused by elastic constriction of blood vessels by releasing anti angiogenic drugs. However, there are still implants after surgery, which may cause problems such as in stent restenosis (ISR) and thrombosis. Although drug-eluting balloons do not have implants and can hinder intimal hyperplasia without leaving permanent implants by delivering anti proliferative drugs to the local vascular wall, they also cannot provide sustained support and are prone to vascular dissection and elastic retraction.
In response to current technological limitations, Juzheng Medical has taken the lead in introducing "photocrosslinking technology" into the field of coronary artery treatment worldwide and successfully developed coronary artery remodeling catheters.
In terms of innovation, this product induces photocrosslinking reactions in the extracellular matrix of vascular media by precisely controlling the transmission and release of laser, constructing an in-situ micro crosslinked support structure, effectively reducing the occurrence of vascular dissection and elastic shrinkage of the tube wall. Moreover, as this technology is based on the self crosslinking of extracellular matrix, there are no implants left after the catheter is withdrawn, which can maintain the natural anatomical structure and physiological characteristics of the vascular lumen. Patients do not need to take antiplatelet drugs for a long time, and can truly achieve "intervention without implantation".
Innovation carries the dual mission of improving patients' quality of life and promoting industry progress. From the expansion of technological boundaries to the innovation of treatment concepts, since its establishment in 2021, Juzheng Medical has always taken solving clinical pain points as the first starting point and is committed to developing treatment plans that are truly suitable for Chinese and even global patients. Looking ahead to the future, Juzheng Medical will take "innovation lights up life" as its mission, continue to cultivate clinical needs, use "Chinese original" to benefit global patients, and define the infinite possibilities of vascular intervention.
About Matrix
Future oriented innovation intervention technology platform
Hangzhou Matrix Medical Technology Co., Ltd. (hereinafter referred to as' Matrix Medical ') was founded in 2021 and is the first high-tech enterprise in China to master intravascular photochemical cross-linking technology. It focuses on the research and development and production of innovative medical devices in the field of vascular intervention, and is committed to the research and development of a series of natural vascular stent products without implantation. Juzheng Medical is headquartered in Hangzhou Future Science and Technology City and has a production base of 1700 square meters in Anji.
In the future, Matrix Medical will continue to adhere to the concept of "intervention without implantation", develop new interventional medical devices, take solving clinical pain points as the first starting point, truly provide new solutions for patients undergoing vascular intervention therapy, and actively promote the research and development of innovative technologies to further promote the development of the field of vascular intervention therapy.